Vascular grafts
Expanded polytetrafluoroethylene (ePTFE) is a commonly used material for prosthetic implant applications. It’s mechanical strength, impermeability to blood and inertness
Trauma Fixation
Gas plasma precision cleaning, sterilization and surface activation of orthopedic implants promotes biocompatibility in a single, highly reproducible process step
Tissue Scaffold
NH3 plasma enhances cell affinity to porous polyactone scaffolds. Studies show cell seeding efficiency can be maintained above 99%, which is better than that achieved by prewetting with ethanol.
Stents and Shunts
For a lubricious coating to be effective on a medical device it must adhere to the substrate well enough that it doesn’t come off during usage. Herein lays the technology.
Spinal implant
Polyetheretherketone (PEEK) is a preferred material for vertebral implants due to its biocompatibility, physical properties, and above all its radiolucency. It is a semi-crystalline thermoplastic and therefore prone to absorption of cleaning solvents
Pacemakers
Plasma treatment of pacemakers and implantable cardioverter defibrillators ensures hermetically sealed devices and reliably bonded subcomponents
Microfluidics
The function of microfluidic devices are greatly enhanced by plasma. Microchannels on clinical diagnostic devices are made “wettable” to bio-fluids without having an effect on the properties of the analyte itself.
Laboratory wear
Materials used for laboratory containers (test tubes, vials, flasks, etc.) need to be chemically inert, shatter proof, non leachable, and most often require optical clarity. Glassware fulfills most of these criteria
Joint Replacement
Gas plasma precision cleaning, sterilization and surface activation of orthopedic implants promotes bio compatibility in a single, highly reproducible process step
Intraocular Lens
The replacement procedure of diseased or damaged natural eye lenses by artificial intraocular lenses (IOLs) requires an incision in the eye of just 2 – 3 millimeters. IOLs are manufactured using soft polymers
Intraoccular delivery device
Foldable intraocular lenses (IOL’s) can be implanted through <3mm incisions in the eye with the aid of an intraoccular delivery device. To assist in the mobility
H202 Sterilization
The increasing importance of infection control in life science industries is placing greater focus on sterilization technologies. New regulatory forces are generating industry specific criteria.
Guidewires
Lubricious coatings are best described as “slippery when wet” yet non-slippery when dry. They facilitate the insertion and manipulation of guide wires
Defibrillators
Artificial cardiac rhythm systems are complex devices with sub components requiring robust integration. Pacemakers and defibrillators are composed of electrode wires and sensors
Decontamination
When comparing the many different methods of sterilization, plasma sterilization has the distinct advantage in that it decontaminates as well. Decontamination means that the proteinacious
Contact Lens
Since the recent advent of silicone hydrogels, contact lenses can now be fabricated from a soft material with extremely high oxygen permeability. Unfortunately, hydrogels are also inherently
Cell Cultureware
Platforms for cell culture plates and cellular matrices are predominantly fabricated from synthetic polymers. While such materials are ideally inert, mechanically stable, and low cost for this industry
Catheters
PVA TePla’s low pressure or atmospheric plasma systems are used to improve wettability, bondability, printability and also to improve uniformity and lifetime of catheter coatings.
Biosensors
Gas plasma provides surface conditioning of in vitro diagnostic platforms prior to the deposition of the bio sensor materials. Conditioning may simply be precision cleaning of the substrate at the molecular level
Biochemical Assays
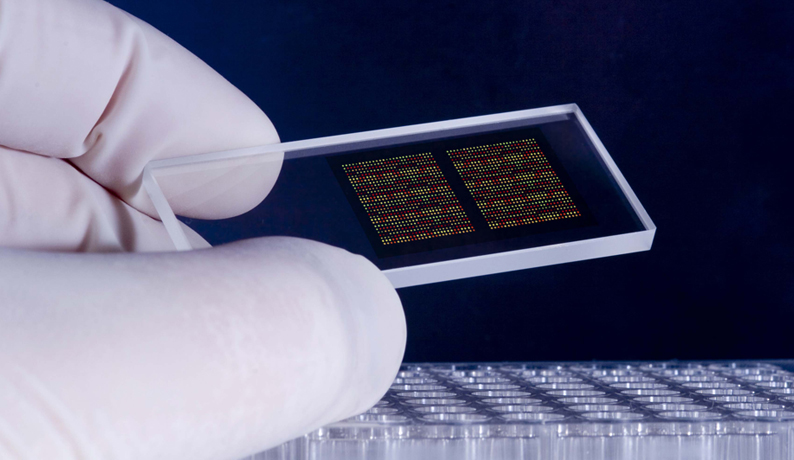
Microarray technology has accelerated the rate of biomolecular research by enabling an enormous number of experiments to be conducted in parallel. This is achieved by immobilizing arrays of probe biomolecules